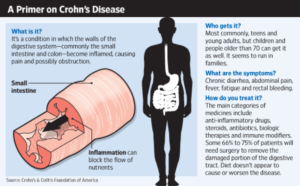
Crohn’s disease is an inflammatory bowel disorder that causes ulcers and sores throughout the digestive tract and is characterized by abdominal pain, diarrhea, and bloody stools. A painful disease with no known cure, Crohn’s disease is most common in young men between the ages of 15 and 30. Treating Crohn’s disease means management of symptoms, and in severe cases, surgery to remove part of the bowel.
According to the Crohn’s and Colitis Foundation of America, 1.6 million Americans suffer from inflammatory bowel diseases; including around 700,000 with ulcerative colitis or Crohn’s disease.
Understanding Crohn’s Disease
Crohn’s disease is often confused with ulcerative colitis (UC). They share some of the same symptoms. However, Crohn’s disease affects the entire digestive tract from the mouth to the anus and in all layers of tissue, whereas, UC is centered in the large intestine affecting the colon’s inner lining.
Pathophysiology of Crohn’s Disease
Although the exact cause of Crohn’s disease is unknown, the immune system, GI flora, genetics, and infections play a role. Factors like diet, medications, and infections, combined with an abnormal inflammatory response or genetic abnormalities may contribute to Crohn’s disease.
In a normal digestive tract without Crohn’s disease, external factors trigger an inflammatory response1, directing white blood cells to the affected area to destroy antigens. Activating certain cells, called T-helper cells or Th1 cells, produces cytokines, a proinflammatory substance which initiates the removal of antigens from the body.
Crohn’s disease causes a dysregulation of this inflammatory reaction, creating an imbalance in homeostasis which causes problems detoxifying, as well as tissue injury.
Eventually, the tissue damage transforms into an ulcer, worsening until forming granulomas – walls of immune cells built around antigens it cannot remove. Granulomas invade all layers of tissue, affecting a significant portion of the digestive tract and lymph nodes. White blood cells continue the onslaught causing more damage and leading to colon atrophy. The bowel wall thickens and narrows, resulting in bowel obstruction, weight loss, stomach pain, fatigue, inflammation of the mouth and lips, and abdominal bloating. Crohn’s disease hinders nutrient absorption and may even slow growth in children.
Current Treatments for Crohn’s Disease
With no cure for Crohn’s disease, patients must rely on medications for symptom control. Unfortunately, pharmaceuticals frequently used for treating Crohn’s disease have adverse side effects of their own:
- Anti-inflammatories – headaches, nausea, vomiting, and heartburn.
- Corticosteroids – swelling, night sweats, insomnia, tingling in the extremities, muscle pain and weakness.
- Immunosuppressants – can cause lymphoma, skin cancer, tuberculosis, pneumonia, liver cancer, and fatal infections.
Long-term use of other Crohn’s disease medications such as antibiotics, antivirals, anti-diarrheal, and pain relievers can cause hypertension, diabetes, bone diseases like osteoporosis, glaucoma, immune suppression, and seizures, as well as pancreas, liver, and kidney problems.
Cannabis: An Alternative Treatment for Crohn’s Disease
Medications help in controlling the symptoms of Crohn’s disease, but side effects like these can do more harm than good. Through cannabis research, scientists are learning how cannabinoids may be a less damaging way to treat Crohn’s disease.
The endocannabinoid system has two primary receptors, CB1 and CB2. Although both receptors are found throughout the body, CB1 receptors are predominantly located in the central nervous system – the brain and spinal cord; while CB2 receptors are found in the immune system and the hematopoietic system which includes bone marrow, lymph nodes, the spleen, and thymus.
CB1 receptors regulate homeostasis and control functions like stress, anxiety, aggression, mood, arousal, appetite, sleep, blood pressure, cardiac performance, motor control, pain perception, and even behavior. CB2 receptors regulate the inflammatory process by controlling the release of cytokines produced by the immune cells, which is integral to Crohn’s disease. Activating these receptors results in pain relief and anti-inflammatory effects.
Endocannabinoids vs. Phytocannabinoids
Anandamide and 2-Arachidonoylglycerol (2-AG) are endocannabinoids produced by the human body. Anandamide binds to CB1 receptors and acts as an agonist to CB2, while 2-AG binds with both receptors. Both act as signaling molecules, binding with receptors to produce therapeutic effects and promote homeostasis. Unfortunately, enzymes degrade endocannabinoids quickly. Thus, their effects are short-lived.
Phytocannabinoids (cannabinoids from plants) bind with the same receptors and exert the same therapeutic benefits of endocannabinoids. Tetrahydrocannabinol (THC) can produce psychoactive effects by binding to CB1 receptors; however, others like cannabidiol (CBD) do not bind to CB1 receptors, thus creating no psychoactive effect. Researchers believe these phytocannabinoids can significantly benefit people suffering from Crohn’s disease.
THC: Anti-Inflammatory and Anti-Motility Effects
THC can reduce inflammation in the gut2 and control diarrhea by binding with the CB2 receptors, essentially sending Th1 cells into a controlled death, resulting in the reduction of cytokines and proinflammatory cells being forwarded to the inflamed walls of the GI tract. Additionally, THC binds to the CB1 receptors of the enteric nervous system (ECS), a mesh-like group of neurons in the gastrointestinal system lining which regulates the GI tract. Activating this receptor reduces the GI tract’s hypermotility, alleviating diarrheal symptoms associated with Crohn’s disease.
CBD: Anti-Inflammatory, Anti-THC Effects
CBD by itself is a very potent anti-inflammatory compound3 that has potent antioxidant benefits4, but when combined with THC, CBD not only prolongs the therapeutic effects, but also reduces the psychoactive effects of THC.
- The “high” associated with cannabis use is produced by activating CB1 receptors; however, by binding to a different part of the receptor, CBD diminishes the receptor’s ability to transmit a signal, effectively inhibiting THC’s psychoactive effects5.
- The cytochrome P450 system6 is a collection of enzymes found in the liver responsible for the metabolic breakdown of drugs, including THC and CBD. Because CBD is a “competitive inhibitor,” competing to bind to the receptors, it slows the breakdown of THC.
CBD: Anti-Motility Effects
CBD reduces inflammatory hypermotility7 associated with inflammatory bowel diseases like Crohn’s disease:
- By inhibiting a particular liver enzyme that breaks down endocannabinoids, CBD supplementation allows anandamide to exert its natural anti-motility and anti-inflammatory effects. CBD will only affect the inflamed area, leaving healthy areas of the gut untouched.
- CBD does not bind to CB1 receptors but can activate the receptors by increasing endocannabinoid levels.
- CBD suppresses muscle contractions induced by a neurotransmitter called ACh.
CBD influences certain opioid receptors by binding with their allosteric, or alternate sites. When activated, opioid receptors8 control motility and secretion. Activating these receptors blocks the contractions of the digestive tract which facilitates digestion. The study proves CBD is an allosteric modulator9 that can alter the shape or structure of the receptor by binding to an allosteric site, ultimately influencing how the stimulus is handled.
More studies are needed to support this theory, but it is encouraging to find CBD can enhance or weaken effects when certain receptors are activated.
CBD Influences Extra-Cannabinoid System Receptor Sites
Cannabinoids also have the potential to exert their anti-inflammatory, analgesic, and antimotility effects through the activation of extra-cannabinoid receptors10 such as:
· Transient receptor potential vanilloid 1 receptors (TRPV1)
TRPV1 receptors, frequently called capsaicin receptors because activation results in a particularly painful, burning sensation, are found all over the gastrointestinal tract and in the mucosal immune system. These receptors act on tissue damage, sending inflammatory mediators to increase the perception of pain. Prolonged activation of the receptors through cannabinoid therapy causes desensitization brought on by calcium influx into the cells, activating the calmodulin and calcineurin signaling pathways, desensitizing the calcium channels themselves, and resulting in analgesia (the inability to feel pain).
Activation of the TRPV1 receptors also results in anti-inflammatory effects by releasing an antibody which slows cytokine production and inflammation11. Enteric nerve cells release somatostatin, a type of neuropeptide responsible for inhibiting the inflammatory process12.
· Peroxisome proliferator-activated receptors (PPARs)
When activated, these receptors produce anti-inflammatory effects13 by inhibiting the synthesis and release of the proinflammatory cytokines.
CBD binds with PPARs14 to produce anti-inflammatory effects, as well as, regulate the gastrointestinal tract’s neuroimmune axis, which promotes homeostasis of the enteric nervous system. During GI tract injuries, they contribute to the proliferation of proinflammatory cells. CBD inhibits the hyperactivation of the receptors and reduces gut inflammation.

- Orphan G protein-coupled receptors (GPR55)
Playing a role in appetite, weight control, bone reabsorption, diseases like cancer, gastrointestinal motility, secretion, and inflammation, too much stimuli of these receptors increases cancer growth and promotes bone reabsorption, as well as worsens the proinflammatory response15 by increasing cytokine production. CBD acts as an antagonist to the GPR55 receptors16 thus reducing inflammation.
Studies on the Effectiveness of Cannabinoids for Treating Crohn’s Disease
THC and CBD, promising alternatives for treating Crohn’s disease, work together to relieve symptoms, while CBD counteracts negative side effects of THC.
Clinical trials that helped people with Crohn’s disease and inflammatory bowel disease include:
- 21 patients with Crohn’s disease who did not respond to traditional treatments were grouped into two sub-groups; 11 patients received cannabis cigarettes while the other 10 patients received a placebo. Researchers assessed patients’ symptoms and disease activity for ten weeks. While all reported significant improvements in their Crohn’s disease symptoms17, 5 of the 11 patients given cannabis experienced complete remission of Crohn’s disease, with ten showing lab test improvements. Additionally, three cannabis patients eliminated a dependency on steroids without any adverse side effects during the study as well.
- In another study assessing cannabinoids’ impact on chronic inflammatory bowel diseases like Crohn’s disease, 13 individuals were prescribed cannabis therapy for three months. After the trial, all 13 participants reported significant improvement18 in their pain and depression levels as well as in their overall quality of life.
- In a lab study, colitis was induced in rats followed by various forms of CBD treatments. Treatments showed a reduction in colonic inflammation19 as well as improvement in the disease parameters.
These studies show that cannabinoids, particularly CBD, are a proven alternative treatment or at least an adjuvant therapy for Crohn’s disease as proven by researchers. CBD is clearly effective in reducing GI inflammation and other symptoms associated with Crohn’s disease.
References:
1 Leyla J. Ghazi, MD. January 6, 2017.
Medscape, Crohn’s disease.
http://emedicine.medscape.com/article/172940-overview#a4
2 Rudolf Schicho and Martin Storr. December 17, 2013.
The National Center for Biotechnology Information, Cannabis finds its way into treatment of Crohn’s disease.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4076530/
3 Guiseppe Esposito, et al. July 20, 2012
Wiley Online Library, Cannabidiol in Inflammatory Bowel Diseases: A Brief Overview.
https://lirias.kuleuven.be/bitstream/123456789/356043/1/ptr4781.pdf
4 JM Jamontt, et al. June 2010.
The National Center for Biotechnology Information, The effects of Δ9-tetrahydrocannabinol and cannabidiol alone and in combination on damage, inflammation and in vitro motility disturbances in rat colitis.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2931570/
5 RB Laprairie, et al. October 2015.
The National Center for Biotechnology Information, Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4621983/
6 LM Bornheim, et al. March 24, 1993.
The National Center for Biotechnology Information, Characterization of cannabidiol-mediated cytochrome P450 inactivation.
https://www.ncbi.nlm.nih.gov/pubmed/8466552
7 R Capasso, et al. July 2008.
The National Center for Biotechnology Information, Cannabidiol, extracted from Cannabis sativa, selectively inhibits inflammatory hypermotility in mice
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2451037/
8 Peter Holzaer. June 5, 2009.
The National Center for Biotechnology Information, Opioid receptors in the gastrointestinal tract.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3163293/
9 M Kathmann, et al. February 2006.
The National Center for Biotechnology Information, Cannabidiol is an allosteric modulator at mu- and delta-opioid receptors.
https://www.ncbi.nlm.nih.gov/pubmed/16489449
10 AA Izzo and KA Sharkey. April 2010.
The National Center for Biotechnology Information, Cannabinoids and the gut: new developments and emerging concepts.
https://www.ncbi.nlm.nih.gov/pubmed/20117132
11 Fumio Tsuji and Hiroyuki Aono. August 2012.
The National Center for Biotechnology Information, Role of Transient Receptor Potential Vanilloid 1 in Inflammation and Autoimmune Diseases.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3763671/
12 J. D. Van Bergeijk and J. H. P. Wilson. December 1997.
The National Center for Biotechnology Information, Somatostatin in inflammatory bowel disease.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2365882/
13 Jihan Youssef and Mostafa Badr. July 29, 2004.
The National Center for Biotechnology Information, Role of Peroxisome Proliferator-Activated Receptors in Inflammation Control.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC551585/
14 Daniele De Filippis, et al.
December 6, 2011. The National Center for Biotechnology Information, Cannabidiol Reduces Intestinal Inflammation through the Control of Neuroimmune Axis.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3232190/
15 Mirko Lanuti, et al. May 13, 2015.
Public Library of Science, Activation of GPR55 Receptors Exacerbates oxLDL-Induced Lipid Accumulation and Inflammatory Responses, while Reducing Cholesterol Efflux from Human Macrophages.
http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0126839
16 Rudolf Schicho and Martin Storr. December 2012.
The National Center for Biotechnology Information, A potential role for GPR55 in gastrointestinal functions.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3660623/
17 T Naftali, et al. October 2013.
The National Center for Biotechnology Information, Cannabis induces a clinical response in patients with Crohn’s disease: a prospective placebo-controlled study.
https://www.ncbi.nlm.nih.gov/pubmed/23648372
18 A Lahat, et al.November 17, 2011.
The National Center for Biotechnology Information, Impact of cannabis treatment on the quality of life, weight and clinical disease activity in inflammatory bowel disease patients: a pilot prospective study.
https://www.ncbi.nlm.nih.gov/pubmed/22095142
19 Rudolf Schichoa and Martin Storrb. March 12, 2012.
The National Center for Biotechnology Information, Topical and Systemic Cannabidiol Improves Trinitrobenzene Sulfonic Acid Colitis in Mice.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3668621/

Leave a Reply
You must be logged in to post a comment.